Updated March 2, 2021 with full published study link.
Chronic low back pain (LBP) is one of the most prevalent and challenging musculoskeletal conditions and is the leading cause of disability among men and women today.[1] Lumbar radiofrequency ablation (RFA) is a commonly used treatment, but RFA can be followed by a return or worsening of pain that requires re-treatment or lead to multifidus atrophy & disc degeneration.
Goal of the study: Evaluate PNS for Low Back Pain in Patients Following RFA
PNS was evaluated for the treatment of chronic low back pain in patients following radiofrequency ablation. The goal of the study was to see if PNS could offer back pain patients a motor-sparing treatment alternative to RFA.
Study Demonstrates PNS as a Motor-Sparing Treatment Option in Post-RFA Patients
SPR Therapeutics Inc. recently shared the results of a back pain study “Percutaneous PNS of the Medial Branch Nerves for the Treatment of Chronic Axial Back Pain in Patients Following Radiofrequency Ablation” at the American Society of Regional Anesthesia and Pain Medicine’s 19th Annual Pain Medicine Meeting and the North American Neuromodulation Society’s 2021 Annual Meeting. The study evaluated individuals at least six months post-RFA with a return of low back pain who underwent bilateral implantation of the SPRINT PNS System’s MicroLeads (percutaneous open-coil PNS leads) for up to 60 days targeting the medial branches of the dorsal ramus in the center of the region of pain. Treatment involved stimulation of the medial branch nerves for 6-12 hours/day for up to 60 days. Subjects continued to participate in most of their normal activities. Study participants returned for long-term follow-up visits after the leads were withdrawn using gentle traction.
- The study objective: Determine the potential use of percutaneous peripheral nerve stimulation (PNS) as a motor-sparing treatment alternative to repeat RFA for chronic low back pain patients.
- Key study takeaway: Percutaneous PNS of the medial branch nerves may provide a non-destructive, motor-sparing treatment for chronic axial back pain.
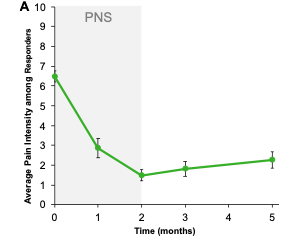
PNS treatment using the SPRINT PNS System resulted in clinically significant reductions in average pain intensity, disability and pain interference.
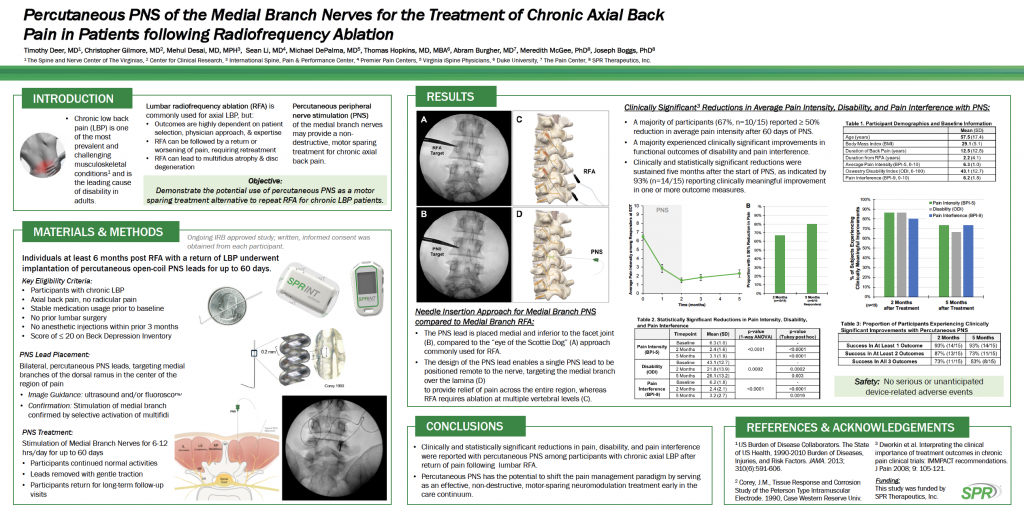
A majority of the study participants (67%, n=10/15) reported ≥ 50% reduction in average pain intensity after 60 days of PNS. A majority also experienced clinically significant improvements in functional outcomes of disability and pain interference. Clinically and statistically significant reductions were sustained five months after the start of the PNS treatment, as indicated by 93% (n=14/15) reporting clinically meaningful improvement in one or more outcome measures.
See the graphs below for the two and five-month results for pain intensity, disability and pain interference.
Conclusion: PNS Treatment of Low Back Pain in Patients Following RFA Has the Potential to Shift the Pain Management Paradigm
PNS has the potential to shift the pain management paradigm and may provide significant reductions in pain, disability and pain interference. The study’s collective results show that PNS can serve as an effective, non-destructive, motor-sparing neuromodulation treatment early in the care continuum. The clinically and statistically significant reductions in pain, disability and pain interference show the effectiveness of PNS as a treatment option for patients with chronic axial low back pain after the return of pain following lumbar RFA. Given the denervation of the multifidus following RFA, serious consideration should be given to the preferential use of medial branch stimulation prior to RFA.
Study leads: Timothy Deer, MD; Christopher Gilmore, MD; Mehul Desai, MD, MPH; Sean Li; MD; Michael DePalma, MD; Thomas Hopkins, MD, MBA; Abram Burgher, MD; Meredith McGee, PhD; and Joseph Boggs, PhD.
[1] https://www.cdc.gov/acute-pain/low-back-pain/index.html
https://www.sprtherapeutics.com/outsmart-pain-2020-landing-page-5/


