
Company Contact
Michelle McDonald
Vice President, Marketing
mmcdonald@sprtherapetuics.com
+1(651) 955-7591
Media Contact
Dave Folkens
dfolkens@sprtherapeutics.com
+1(612)978-6547
If you use or repurpose any of the materials below, please cite SPR Therapeutics, Inc. as the source.
All resources remain copyright of SPR Therapeutics.
Fact Sheets and Brochure
SPR Therapeutics Company Fact Sheet
SPR Therapeutics is a privately-held neurostimulation medical device company focused on developing, manufacturing and commercializing non-opioid, minimally invasive pain treatment options that improve quality of life.
SPRINT PNS System Overview
The SPRINT Peripheral Nerve Stimulation (PNS) System is the only percutaneous PNS device FDA cleared for both chronic and acute pain, including post-operative and post-traumatic pain.
SPRINT PNS System Brochure
The SPRINT PNS System marks an innovative shift in the treatment of pain. The breakthrough treatment is proven to provide significant and sustained relief from chronic pain and works by selectively stimulating targeted peripheral nerve fibers.
Logos


Images
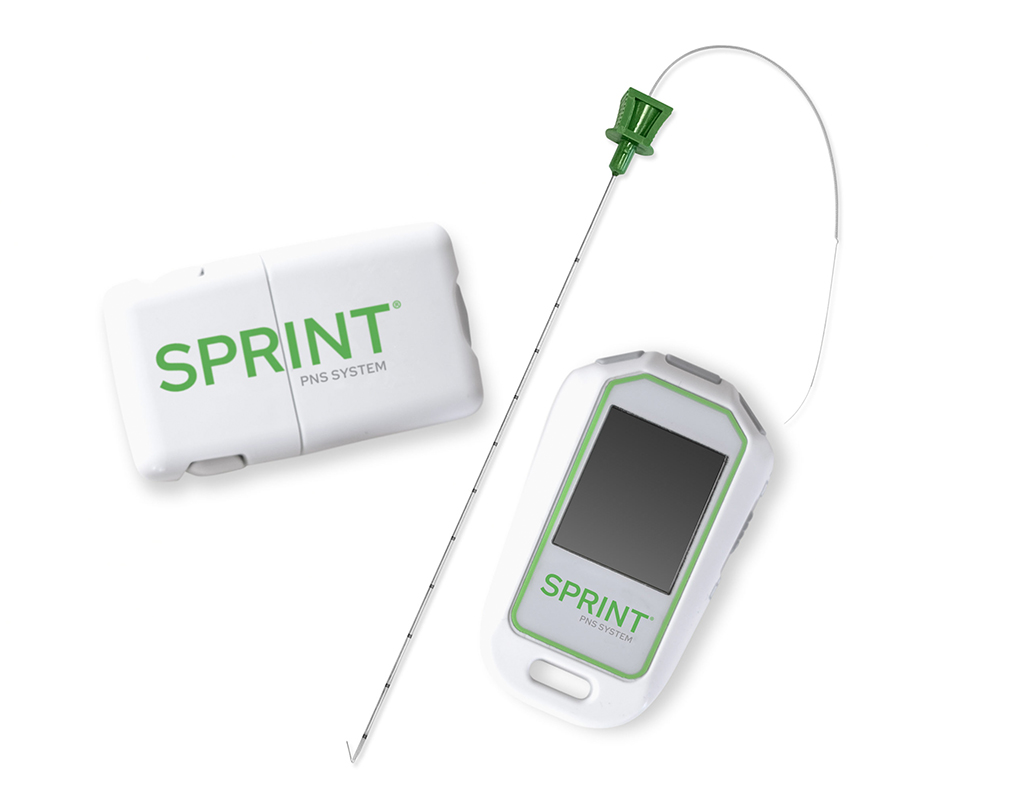
The SPRINT PNS Generator and Remote Control

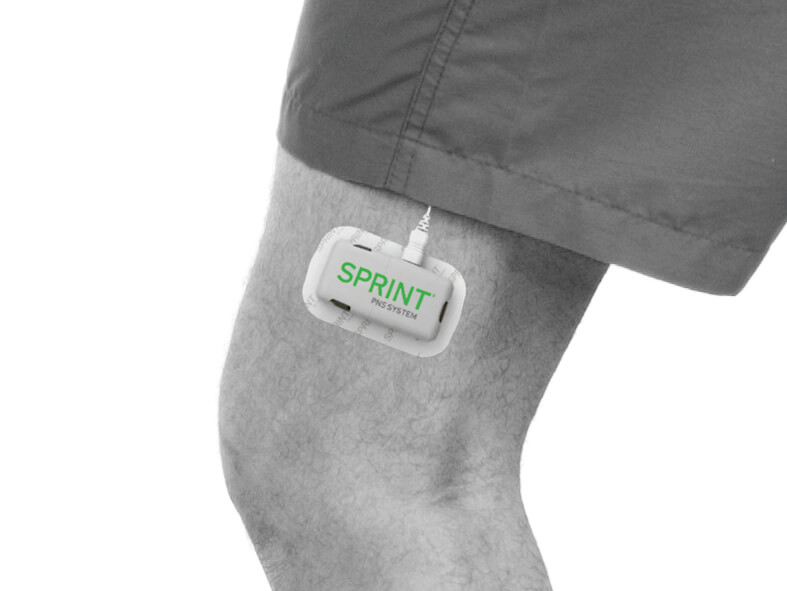
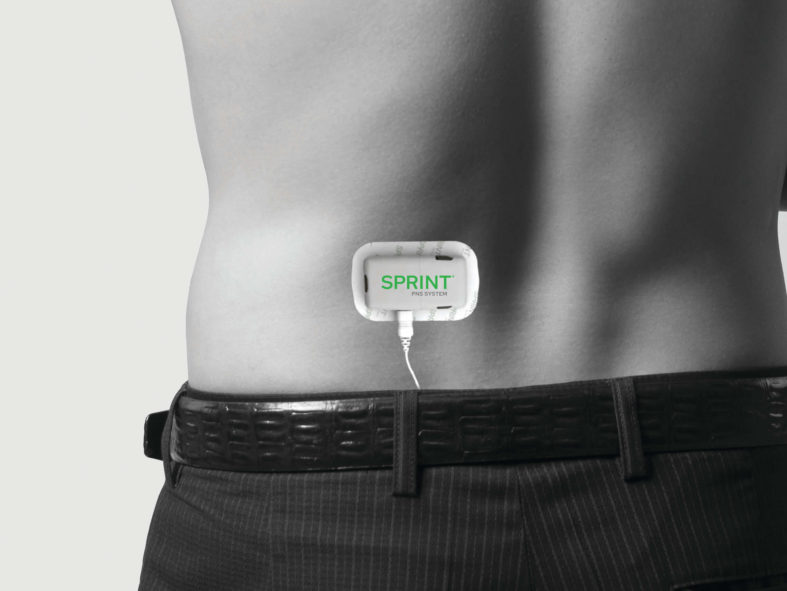
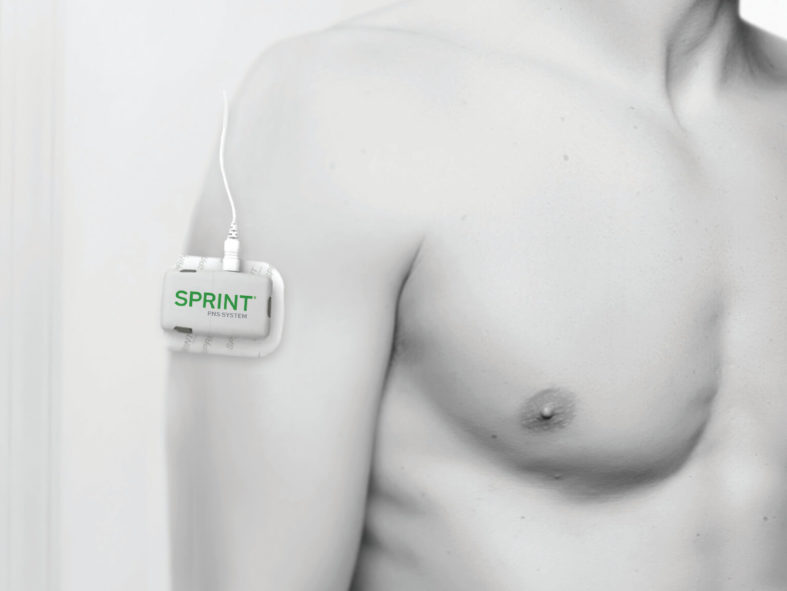
Examples of Pulse Generator Placement
The MicroLead™
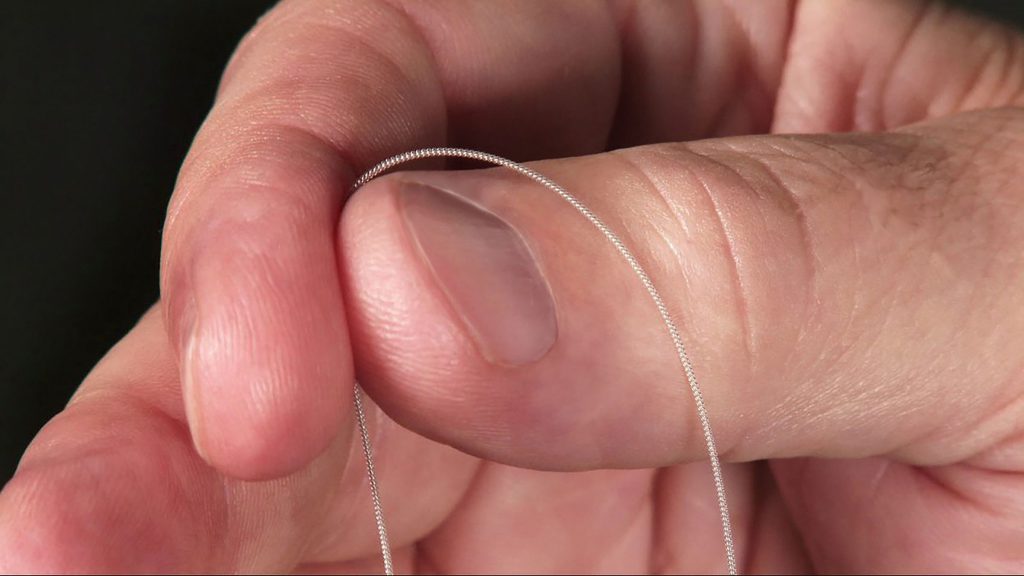


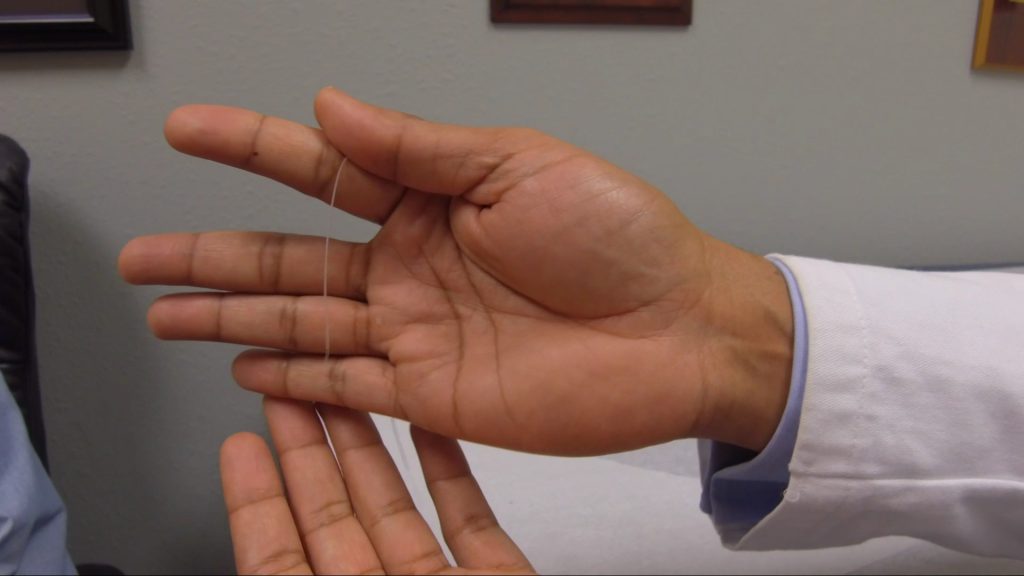
Image Caption
The SPRINT® PNS System is a percutaneous peripheral nerve stimulation (PNS) device FDA cleared for both chronic and acute pain. It works by implanting a thin lead wire called a MicroLead™, typically under image-guidance, targeting a peripheral nerve. The placement does not require surgery or incisions and is typically conducted as an outpatient procedure under local anesthesia. The MicroLead is attached to a wearable external generator that sends small electrical pulses to the nerve for up to 60 days. Patients can manage the stimulation level using a Bluetooth-enabled remote control. After 60 days, the MicroLead is withdrawn in the physician’s office. For additional information regarding safety and efficacy visit SprintPNS.com
Photo credit: SPR Therapeutics, Inc.
Videos
SPRINT PNS Overview
- Patient-preferred, low risk alternative to more invasive treatments
- Efficacy and outcomes
- Patient and physician testimonials
SPRINT PNS B-Roll
- Animation of placement and withdrawal of SPRINT PNS System
- SPRINT PNS System components: MicroLead, generator and remote control
- Patient testimonial and physician exam

