
Short-Term Treatment, Designed for Long-Term Relief.™
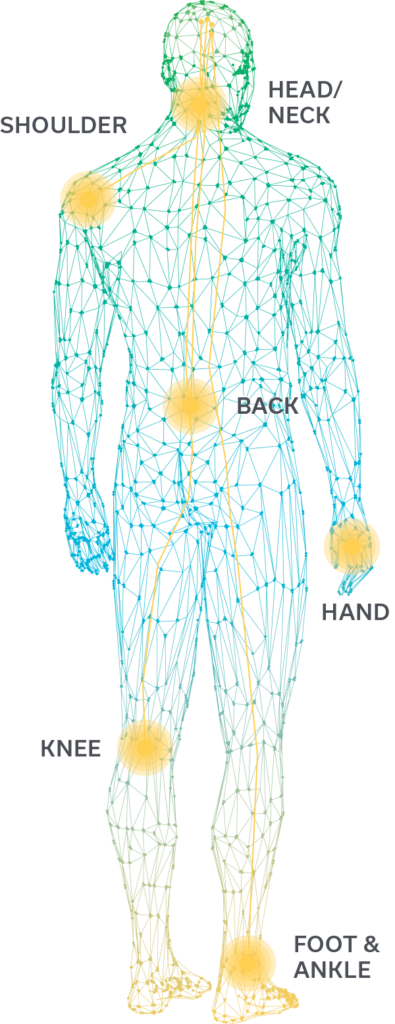
From low back and knee pain to persistent headaches, pain can keep you from fully participating in the life you want. It doesn’t have to stay that way. Introducing the SPRINT® Peripheral Nerve Stimulation (PNS) System.
- 60-day treatment
- Drug-free
- Surgery-free
- Incision-free
- No permanent implant
The SPRINT PNS System has been used to treat:
- Chronic back pain
- Chronic head & neck pain
- Non-operable joint pain
- Post-operative pain
- Post-amputation pain
- Pain due to nerve trauma

How does SPRINT PNS work?
During the 60-day treatment period, the SPRINT PNS System sends tiny electrical pulses through the MicroLead placed near the target nerve. This stimulation is believed to interrupt the pain signals, boost the healthy non-pain signals, and help rebalance the information the brain receives.

Over 70% of patients in clinical studies reported significant & sustained pain relief

“Since the device was implanted, I’ve been able to go on hikes again. I haven’t had to take an opioid pill even one time. The result has been unbelievable.”
— Wayne (back pain patient)






