One of the most prevalent and challenging musculoskeletal conditions, chronic low back pain (LBP), is a leading cause of disability in adults.[1]
The SPRINT PNS System offers a motor-sparing 60-day peripheral nerve stimulation (PNS) treatment option for chronic axial low back pain. SPRINT PNS is designed to be used by pain physicians earlier in the treatment continuum than conventional neurostimulation.
Recently, Dr. Christopher Gilmore, an SPR Consultant, and colleagues sought to better understand how chronic low back pain patients who were recalcitrant to multiple non-surgical treatments would respond to 60-day PNS of the medial branch nerves. [View the full study].
“A lot of patients are opposed to surgical procedures, or at the least, would prefer to avoid undergoing invasive and permanent treatments,” Gilmore said. “Because of the non-surgical nature of temporary percutaneous PNS, we wanted to get in front of patients like this and characterize their responses so as to be able to improve our conversations with patients, and thereby help more individuals with chronic back pain.”
Can Medial Branch PNS Work for Patients Opposed to Surgical Treatments?
Patients suffering from low back pain and averse to invasive or destructive procedures may find temporary PNS a good option. Gilmore, et al., chose patients with broad eligibility criteria in an effort to characterize their response to a 60-day medial branch PNS treatment.

Key eligibility criteria included:
- Subjects with chronic axial LBP (≥3 months) without radicular pain
- Stable medication usage for ≥ 1 month prior to baseline
- No prior lumbar surgery or RFA within prior 6 months
- No anesthetic injections within prior 3 months
- Score of ≤20 on Beck Depression Inventory
Participants were treated with the SPRINT PNS System using ultrasound and/or fluoroscopy guidance to place bilateral, percutaneous PNS leads targeting the medial branches of the dorsal ramus centered in the region of pain.

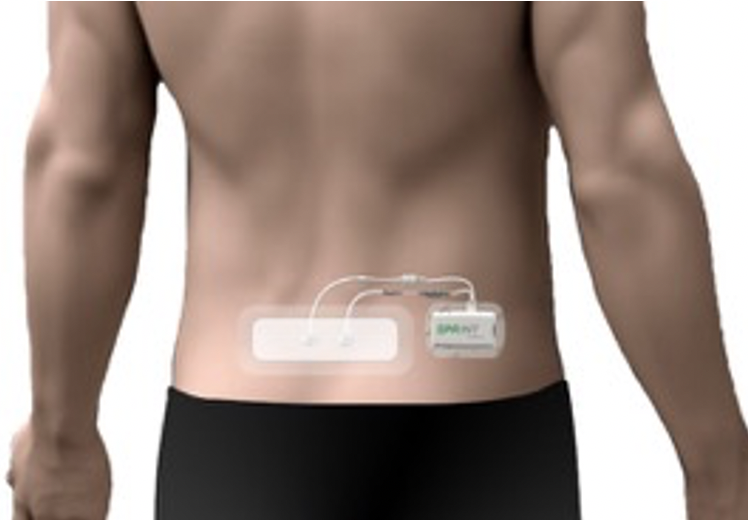
Stimulation of the medial branch was confirmed by selective activation of multifidi. Participants received stimulation for 6–12 hours a day for up to 60 days and continued most normal activities throughout the treatment period. They also participated in long-term follow-up visits up to 12 months post-treatment.
Medial Branch PNS Provided Significant Reductions in Pain Intensity and Interference
The majority of participants who completed the Primary Endpoint and long-term follow-up visits reported clinically meaningful and statistically significant reductions in pain, disability, and pain interference. These reductions were sustained for a majority of participants through 14 months from start of treatment.
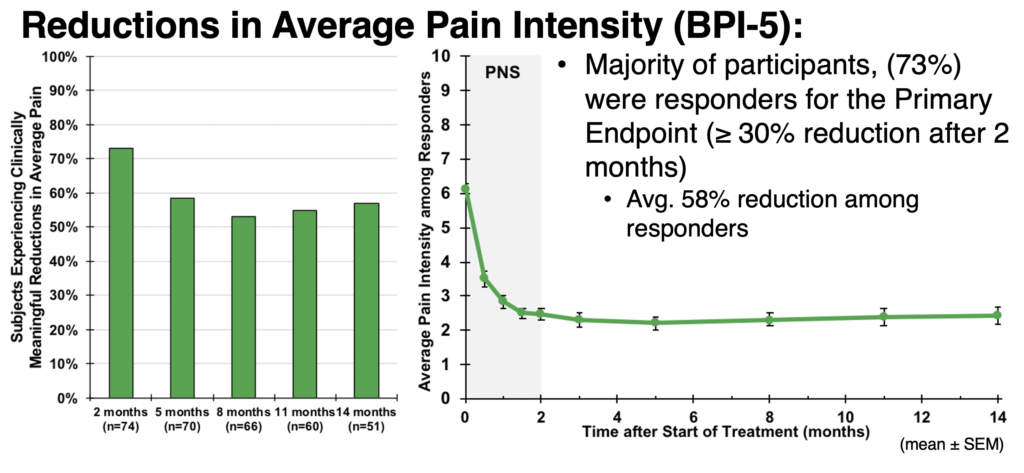
Reductions in Average Pain Intensity (BPI-5)
A total of 73% of participants were responders to the PNS treatment reporting ≥ 30% pain intensity reduction after two months; responders experienced, on average, a 58% reduction in pain intensity. At 14 months from the start of treatment, 50% of subjects (25 of 50 who have completed follow-up to date) continued to experience meaningful improvements in pain intensity.
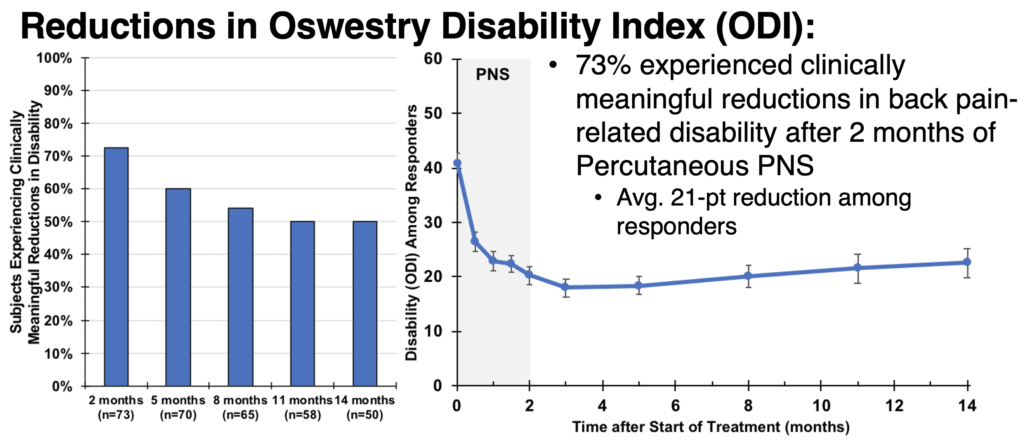
Reductions in Oswestry Disability Index (ODI)
Additionally, 73% of participants experienced clinically meaningful reductions in back pain-related disability after two months of percutaneous PNS. Responders reported an average 21-pt reduction. At 14 months from the start of treatment, 50% of subjects (25 of 50 who have completed follow-up to date) continued to experience meaningful improvements in disability.
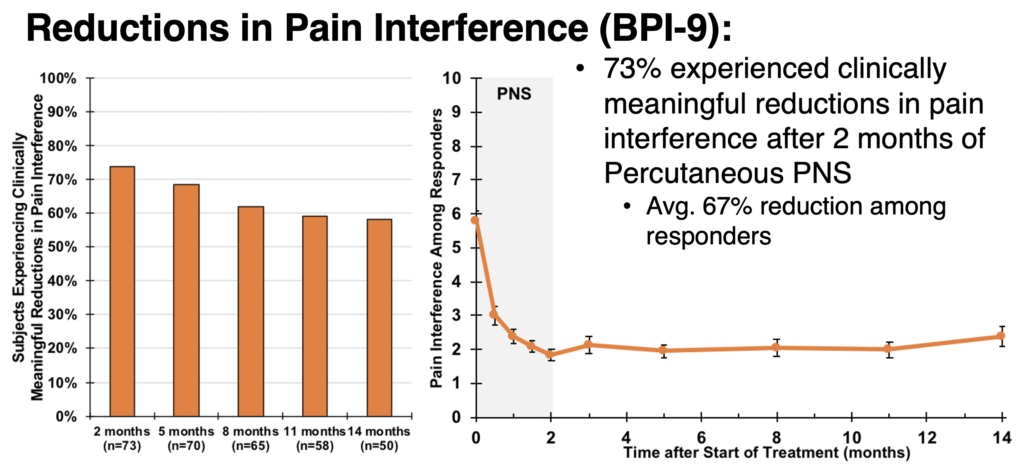
Reductions in Pain Interference (BPI-9)
Following two months of percutaneous PNS, 73% of participants experienced clinically meaningful reductions in the interference of pain with daily activities, as measured by pain interference. Responders reported an average reduction of 67% pain interference. At 14 months from the start of treatment, 58% of subjects (29 of 50 who have completed follow-up to date) continued to experience meaningful improvements in pain interference.
I
Medial Branch PNS Improved Health-Related Quality of Life
In addition to evaluating pain intensity, pain disability, and pain interference, Gilmore, et al., also asked participants to share how the PNS treatment impacted their quality of life (QoL). Using the Patient Global Impression of Change (PGIC) options (Very Much, Much, or Minimally Improved, No Change, Minimally, Much, or Very Much Worse), 90.5% of responders reported quality of life improvements.
Selected subscales from the RAND-36 measure of health-related quality of life were also used to identify statistically significant improvements in quality of life with percutaneous PNS. The table below shows the relevant subscales and marks of improvement in responders (**p < 0.001).
| Selected RAND-36 Subscales | Improvement from Baseline |
|---|---|
| Role Limitations – Physical Health | 128%, 28.1 points** |
| Pain | 62%, 22.1 points** |
| Physical Functioning | 33%, 14.0 points** |
| Energy/Fatigue | 32%, 13.2 points ** |
A Majority of Participants Receiving Medial Branch PNS Reduced or Stopped Opioid Usage
A final promising result from this study was the percentage of participants who either reduced opioid consumption or ceased using opioids for pain relief altogether at the end of the PNS treatment.
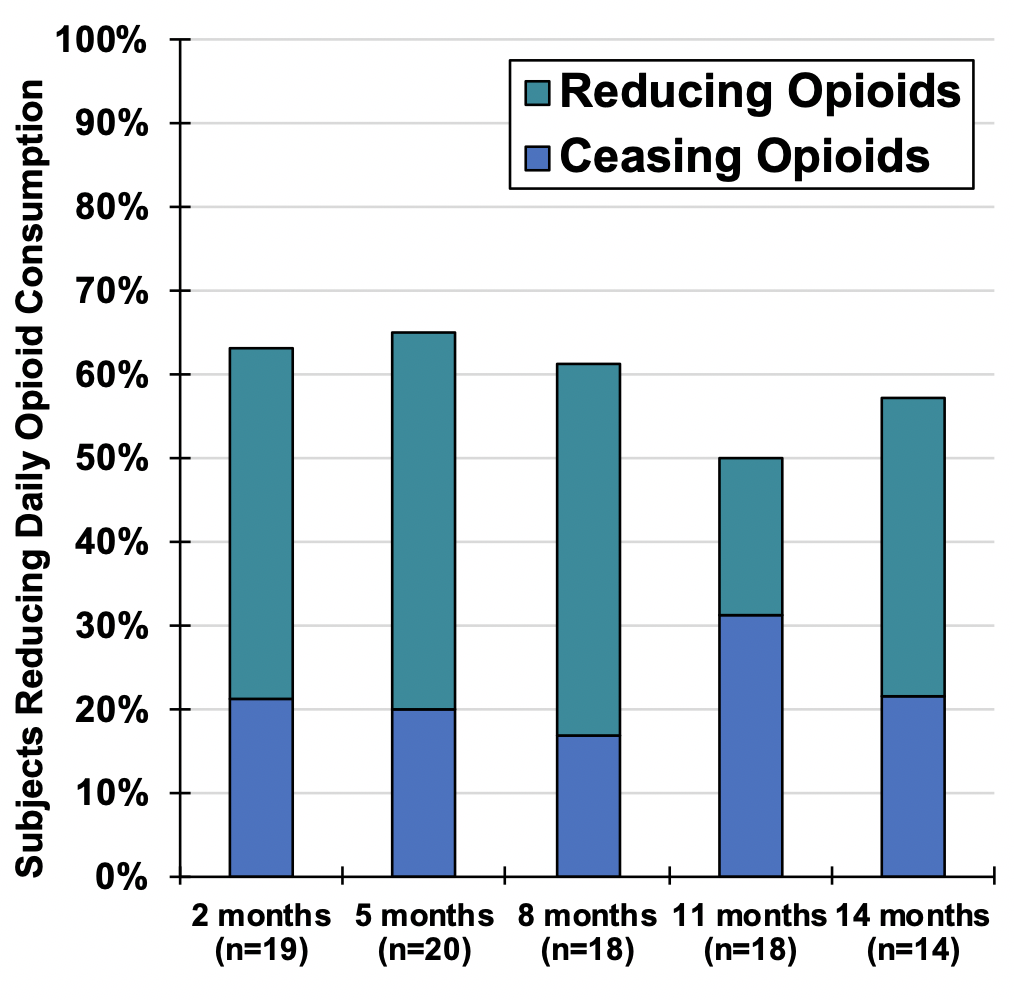
Participants reported:
- 63% reduced opioid usage (with an average 65% reduction noted
- 21% completely stopped using opioids
Opioid reductions were sustained through 14 months from the start of treatment, with 57% of participants reporting opioid reductions and 21% reporting complete cessation of opioids at the end of follow-up.
There were no study-related serious or unanticipated adverse events (AEs).
Adverse events related to the device or procedure that did occur were all non-serious (mild or moderate) and all adverse events were followed to resolution. The most common adverse events were mild skin irritation or pruritis (itching) at the site of the waterproof dressing or stimulator’s hydrogel mounting pad. One participant experienced a superficial skin infection at one lead exit site that was resolved by removal of the lead in the week prior to the end of treatment and use of an oral antibiotic.
Percutaneous PNS is a Promising First-Line Neurostimulation Treatment for Chronic Back Pain
In summary, the majority of the chronic back pain participants in this study reported clinically and statistically significant sustained reductions in pain, disability, and pain interference, as well as improvements in health-related quality of life and reduced usage of (or full cessation of) opioids.
“As we look toward pain treatment options for chronic back pain patients who are uncomfortable with surgery or more invasive procedures, percutaneous PNS offers a promising treatment option early in the care continuum,” said Dr. Christopher Gilmore. “The minimally invasive and temporary nature of the SPRINT PNS System is a winning factor when a patient is concerned about the risks usually associated with surgery as well as the long-term impact on quality of life when considering a permanently implanted system. The data we gathered in this study is a good foundation to help better educate patients while helping them feel comfortable with this treatment approach.”
Given the minimally invasive, non-destructive nature of percutaneous PNS and the significant benefits, percutaneous PNS may provide a promising first-line neurostimulation treatment for patients with chronic axial LBP.
[1] US Burden of Disease Collaborators. The State of US Health, 1990-2010 Burden of Diseases, Injuries, and Risk Factors. JAMA. 2013; 310(6):591-606.
Study and ASPN research abstract poster authors: Christopher A. Gilmore, MD, Mehul J. Desai, MD, MPH, Thomas J. Hopkins, MD, MBA,Sean Li, MD, Michael J. DePalma, MD, Timothy R. Deer, MD, Steven P. Cohen, MD, Meredith J. McGee, PhD, Joseph W. Boggs, PhD

 Multi-Center Study of SPRINT® PNS System for Chronic Low Back Pain Demonstrates Clinically Significant Improvements in Pain and Quality of Life
Multi-Center Study of SPRINT® PNS System for Chronic Low Back Pain Demonstrates Clinically Significant Improvements in Pain and Quality of Life

Leave a Reply