Patients commonly experience acute and chronic phantom limb pain (PLP) and residual limb pain (RLP) after amputation. Up to 80 percent of patients suffer from phantom limb pain post-surgery[1] and 50 percent of those who suffer from phantom limb pain also experience residual pain.[2]
The literature reports the estimated prevalence of PLP and RLP as the following:[3]
- 95% of patients report experiencing amputation-related pain
- 79.9% report phantom limb pain
- 67.7% report residual limb pain
The prevalence of both chronic PLP and RLP is why early prevention and treatment of acute postoperative amputation pain is key. Poorly controlled acute perioperative pain is a significant predictor for developing chronic PLP and RLP. Residual limb pain may contribute to worsening phantom limb pain, increase the potential for developing chronic pain, and earlier treatment is imperative to helping to reduce the incidence of chronic PLP and RLP. Additionally, opioids are still often leveraged perioperatively and postoperatively, sometimes with negative consequences for patients.
Fortunately, various studies have found peripheral nerve stimulation (PNS) to be effective for treating chronic and post-operative amputation pain, including both RLP and PLP. Only recently has a pilot study led by Dr. Denise Lester and Dr. Brooke Trainer evaluated the feasibility of using percutaneous PNS in the early postoperative period.
Evaluating Peripheral Nerve Stimulation for Treating Acute and Subacute Post-Amputation Pain
In a recent study by Lester et al, 16 participants experiencing moderate pain two-to-seven days after transfemoral or transtibial amputation surgery were randomized to receive either eight weeks of percutaneous PNS and standard medical therapy (SMT) or SMT alone. To determine the effect of peripheral nerve stimulation for acute and subacute post-amputation pain treatment, the following primary endpoints were compared between the treatment and placebo groups:
- PLP and RLP intensity scores
- Opioid consumption
Secondary endpoints assessed included readmission rate, length of hospital stay, psychometric scales and functional outcomes.
“Effective management of acute and subacute postoperative pain is particularly challenging following lower extremity amputation,” said Dr. Trainer in a recent discussion about the study along with lead investigator Dr. Lester.
“While PNS has been shown to help manage chronic PLP and RLP, improve functional outcomes, and spare the use of opioids, this is the first time a study has evaluated the feasibility of PNS for acute and subacute use, in the early postoperative period,” said Dr. Lester. “And because patients undergoing amputation often present with multiple preexisting comorbidities, chronic opioid usage, or high predicted acute pain severity, they are quite difficult to treat. The findings in our study are even more important in that they further highlight temporary PNS as an effective non-opioid pain treatment option.”
PNS is feasible in the acute postoperative period and has potential to provide significant reductions in acute PLP and RLP
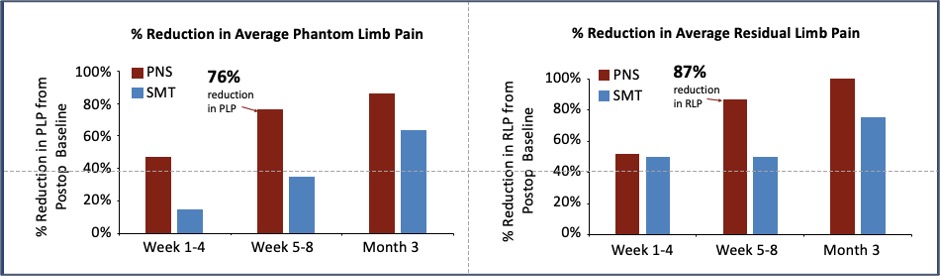
At the end of eight weeks, Lester et al were able to demonstrate the relative efficacy of percutaneous PNS plus SMT compared to SMT alone for acute and subacute post-amputation pain. According to the authors, PNS has the potential to reduce pain intensity and opioid consumption in the acute and subacute post-operative period. At the end of the 8-week PNS treatment period in the study, the following results were observed:
| Study results observed for PNS treatment vs SMT alone | PNS + SMT Group | SMT Alone |
| Average phantom limb pain intensity reductions* | 76% | 29% |
| Average residual limb pain intensity reductions* | 86% | 52% |
| Percentage of subjects using opioids at 8 weeks post-op | 20% | 50% |
| Opioid dose increase (decrease) compared to pre-op levels | (60%) | +200% |
Additionally, the PNS plus SMT group saw lower readmission rates than the SMT alone group. There were no unanticipated serious adverse study-related events, and no infections or lead fractures were reported.
The study authors concluded that short-term PNS is a promising non-opioid treatment in the management of acute and sub-acute postoperative pain following lower extremity amputation and that further larger studies are warranted.
Further Support for PNS as Opioid Alternative for Acute Pain
Of the findings above, the reductions in opioid consumption Lester et al noted — both the number of participants and average daily use — further support the need to study short-term peripheral nerve stimulation as a viable treatment alternative to opioids when treating acute postoperative pain.
In their study, Dr. Lester and colleagues observed the following outcomes regarding opioid usage:
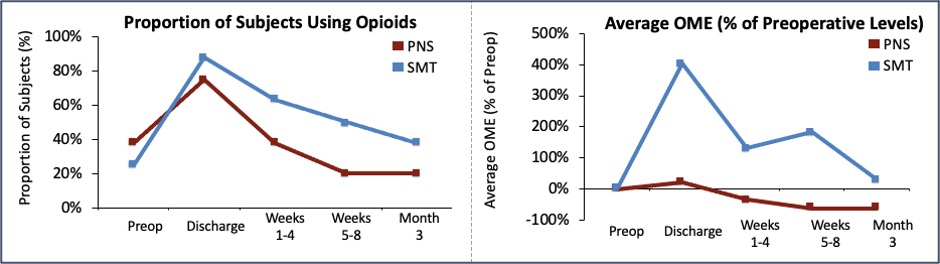
- Though the PNS group had higher average Oral Morphine Equivalents (OME) usage preoperatively (36.1 mg OME in PNS group vs. 7.2 mg OME in SMT group), fewer subjects in the PNS group were using opioids compared to SMT alone at each post-operative time point throughout therapy. At Week 8 (the end of the PNS treatment), one subject (20%, 1/5) in the PNS group was still using opioids compared to 50% (4/8) in the SMT group.
- Usage increased relative to pre-operative levels in the SMT group to 20.3 mg by the end of Week 8 (over 200% increase), while usage decreased relative to pre-operative levels to 13.5 mg (over 60% decrease) in the PNS group.
- Only in the PNS group did Average OME fall below pre-operative levels within the first three months post-amputation.
“Treatment of pain associated with major amputation has traditionally relied on opioid therapies due in part to the diverse challenges these pain conditions present, and the relatively recent entrance of PNS as a non-opioid option,” said Dr. Lester. “We know that long-term opioid use is not beneficial to the patient’s overall health and well-being. Our findings in tandem with the significant reductions in postoperative opioid use and pain following painful orthopedic procedures recently reported by Ilfeld et al in a multi-center RCT highlight the role for short-term peripheral nerve stimulation as a promising alternative to opioids when treating acute postoperative pain.”
Given these data, Dr. Lester encourages consideration of PNS as a non-opioid option, especially when looking at how quickly a patient can succumb to chronic opioid use. According to an analysis by the Centers for Disease Control (CDC) seeking to quantify the transition from acute to chronic opioid use, the largest increments in probability of continued opioid use occurred after the fifth and thirty-first days of consumption.
“Percutaneous PNS is already cleared by the FDA as a treatment option for chronic and acute pain, but it is in the acute or subacute period that we see the greatest risk for long-term opioid usage occur,” noted Dr. Lester. “And with both our findings as well as those presented by Dr. Illfeld and colleagues, I think we have compelling data to be able to say, ‘Percutaneous PNS offers patients a low-risk pain treatment with the potential to reduce the risk of long-term opioid consumption.’ After all, what if, by introducing percutaneous PNS peri-operatively, we could eliminate patients’ need for high opioid doses, or even opioids altogether?”
Conclusion: Short-Term Percutaneous PNS Offers Low-Risk Acute Pain Treatment Option
Phantom limb pain and residual limb pain can negatively impact patients’ health-related quality of life and have shown to be very difficult to manage and treat. The data presented in this pilot study by Drs. Lester and Trainer and colleagues demonstrate the feasibility of employing percutaneous PNS as a non-opioid pain therapy for acute and subacute postoperative pain following lower limb amputation. Additionally, these findings combined with those of Dr. Brian Illfeld and colleagues, highlight the larger promise of short-term PNS as an aid in providing a non-opioid, temporary pain treatment option for acute and subacute postoperative pain.
[1] Erlenwein, J, et al. Clinical updates on phantom limb pain. PAIN Reports. 2021, 6(1):e888
[2] https://www.mayoclinic.org/diseases-conditions/residual-limb-pain/cdc-20447167
[3] Hanyu-Deutmeyer AA, Cascella M, Varacallo M. Phantom Limb Pain. StatPearls. StatPearls Publishing: Treasure Island, FL; 2021 Jan.
The SPRINT PNS System is indicated for up to 60 days for: (i) Symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain; (ii) Symptomatic relief of post-traumatic pain; and (iii) Symptomatic relief of post-operative pain. The SPRINT PNS System is not intended to be placed in the region innervated by the cranial and facial nerves.
Physicians should use their best judgment when deciding when to use the SPRINT PNS System. For more information see the SPRINT PNS System IFU. Most common adverse events are skin irritation and erythema. Results may vary. Rx only.
Important safety & risk information: https://bit.ly/2FU92NH

 Multi-center Study Demonstrates Significant Reductions in Postoperative Pain and Opioid Use Following Peripheral Nerve Stimulation Treatment
Multi-center Study Demonstrates Significant Reductions in Postoperative Pain and Opioid Use Following Peripheral Nerve Stimulation Treatment

Leave a Reply