Cleveland, Ohio – May 17, 2023 – SPR® Therapeutics announced the publication of a comprehensive real-world retrospective data analysis of over 6,100 patients demonstrating significant pain relief following 60-day percutaneous peripheral nerve stimulation (PNS) treatment with the SPRINT® PNS System in the journal Pain Physician.
Real-world data offer important insights into the effectiveness of a treatment in standard clinical practice outside of a controlled research environment. With a broad range of targeted body regions and peripheral nerves treated, this dataset represents the largest published analysis of PNS outcomes to illustrate the associated improvements on pain and quality of life.
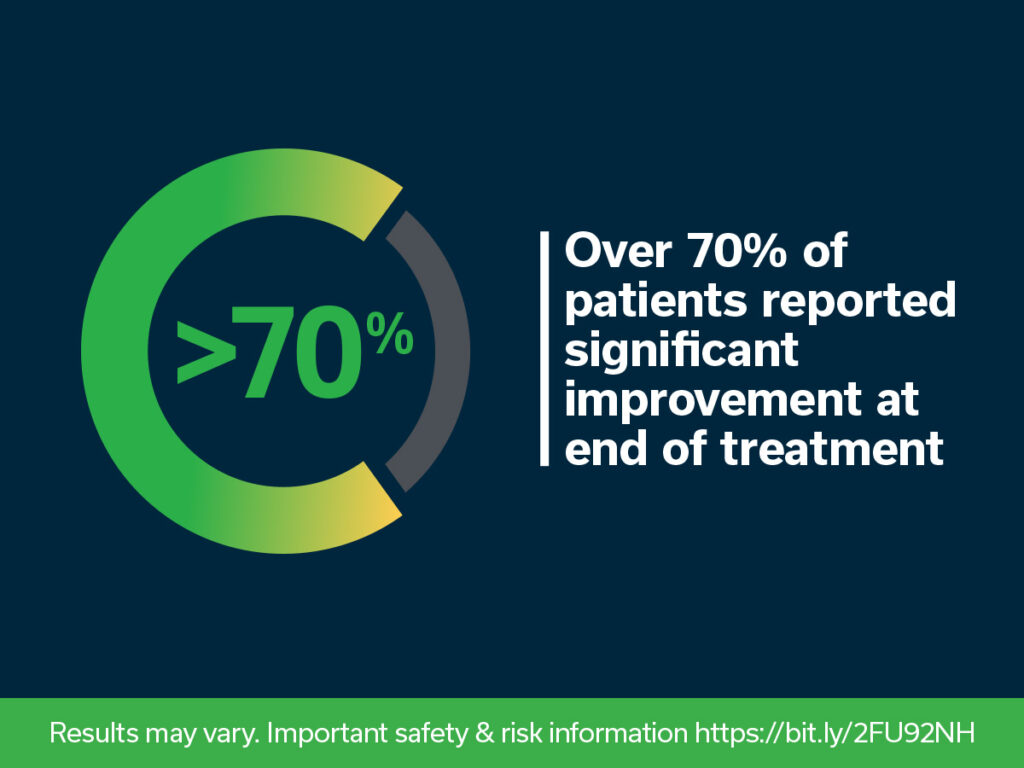
Based on the anonymized data from patients who opted in during their treatment, over 70 percent (4348/6160) of patients were responders with ≥50 percent pain relief and/or improvement in quality of life at the end of treatment. Among these responders, mean percent pain relief was 63 percent, with the majority reporting mild or no pain at the end of treatment. Previously published clinical studies suggest that a majority of responders are likely to experience sustained relief.
The most frequent adverse event reported was skin irritation due to adhesive components with a total rate of adverse events collected from the product complaint database of six percent.
“Seeing impressive real-world results spanning a multitude of nerve targets is significant, especially when considering that these patients may be living with a more challenging list of co-morbidities or conditions than would likely have been allowed for inclusion in prospective clinical trials,” said Marc Huntoon, MD. Dr. Huntoon, SPR’s Director of Medical Affairs, served as the lead author for the study while Director of Pain Management at Virginia Commonwealth University. “In this review of extensive data, there were well over 20 nerve targets in total to address patients experiencing pain throughout the body. The SPRINT PNS treatment performed remarkably well across the gamut of peripheral nerve targets. These data provide the evidence that insurance companies often seek when drafting coverage policy. In this case, the data validates the SPRINT PNS device has similar safety and effectiveness in routine clinical practice compared to clinical studies, complementing prior research further demonstrating that it is not reasonably considered experimental or investigational.”
About the SPRINT PNS System
The SPRINT® PNS System, by SPR® Therapeutics, marks an innovative shift in the treatment of pain. Our breakthrough, 60-day treatment is a First-Line™ PNS option uniquely proposed to recondition the central nervous system to provide significant and sustained relief from chronic pain — without a permanent implant, nerve destruction or the risk of addiction. The system has been studied extensively for low back pain, shoulder pain, post-amputation pain, and chronic and acute post-operative pain, is cleared for use up to 60 days, and is recognized by leading pain management centers. Market research indicates that this breakthrough neuromodulation treatment is a patient-preferred alternative to more invasive options.
The SPRINT PNS System is indicated for up to 60 days for: Symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain; symptomatic relief of post-traumatic pain; symptomatic relief of post-operative pain. The SPRINT PNS System is not intended to be placed in the region innervated by the cranial and facial nerves.
Physicians should use their best judgment when deciding when to use the SPRINT PNS System. For more information see the SPRINT PNS System IFU. Most common adverse events are skin irritation and erythema. Results may vary. Rx only.
For additional information regarding safety and efficacy, visit: SPR Safety Information.
About SPR Therapeutics, Inc.
SPR Therapeutics is a privately held medical device company, providing patients with a non-opioid, minimally invasive pain treatment option. Our SPRINT® PNS System fulfills a critical unmet need for a drug-free, surgery-free option for millions who suffer from chronic pain. Backed by the largest body of clinical evidence in peripheral nerve stimulation for the treatment of pain, SPR has demonstrated commercial demand in untapped peripheral (shoulder and knee) and back pain markets and built an incredibly strong foundation for commercial growth. Headquartered in Cleveland, OH with satellite offices in Chapel Hill, NC and Minneapolis, MN, SPR’s Senior Management team includes experienced industry veterans with nearly 200 years of collective pain market and MedTech expertise, all driven by our purpose – to improve the quality of patients’ lives by providing them with a minimally-invasive, drug-free, surgery-free solution to manage their acute and chronic pain.
More information can be found at www.SPRTherapeutics.com.
SPR Contacts:
Michelle McDonald
Vice President – Marketing
844.378.9108
Dave Folkens
Public Relations
612.978.6547

 SelectHealth Initiates Peripheral Nerve Stimulation (PNS) Coverage to Support Patient Need for Effective Non-opioid Pain Treatment
SelectHealth Initiates Peripheral Nerve Stimulation (PNS) Coverage to Support Patient Need for Effective Non-opioid Pain Treatment

Would like to see a dr in my area being in Santa Maria calif. any help is greatly appreciated
Hello Jerry – Thank you so much for your message about SPRINT PNS! We’d be glad to help answer your questions about the SPRINT PNS System and help you find a physician in your area. Please reach out to our Patient Support team by calling 1-844-378-9108 and selecting option 1 from the menu between 8:00 a.m. and 5:00 p.m. EST. If more convenient, you may email them at patientsupport@sprtherapeutics.com.
Hello, I’m getting ready to receive a dual lead sprint pns for a nerve pain in the bi lateral intercostal nerve area, is there any data from the pns in the intercostal area?
Thank you so much for your comment and becoming a SPRINT patient soon! We are happy to share some published data that might be of interest to you, but please direct any specific questions that you may have to your physician about your own medical situation and likelihood of success. We do have some data on intercostal pain – if you refer to the real world retrospective study: https://www.painphysicianjournal.com/current/pdf?article=NzY1OQ%3D%3D&journal=152
We would direct your attention to Fig. 1, which shows a subset of patients with intercostal pain that experienced greater than 50% pain relief and/or clinically significant improvement in Patient Global Impression of Change. Next, there was a case study that highlights two patients with intercostal neuralgia who experienced significant and sustained relief with SPRINT targeting the intercostal nerves: https://www.painmedicine-casereports.com/current/pdf?article=NTE3&journal=32