
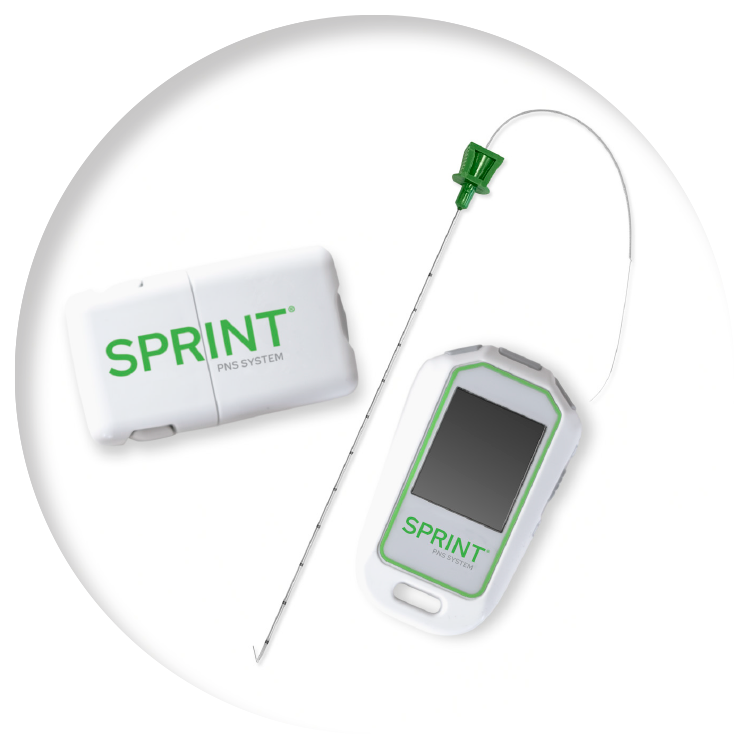
A Breakthrough in Pain Management
The SPRINT® PNS System, by SPR® Therapeutics, marks an innovative shift in the treatment of pain. Our 60-day First-Line PNS™ treatment is proven to provide significant and sustained relief from chronic pain and works by selectively stimulating targeted peripheral nerve fibers.
- Non-destructive, motor-sparing
- No permanent implant
- Minimally-invasive
- Drug-free
Clinically proven.
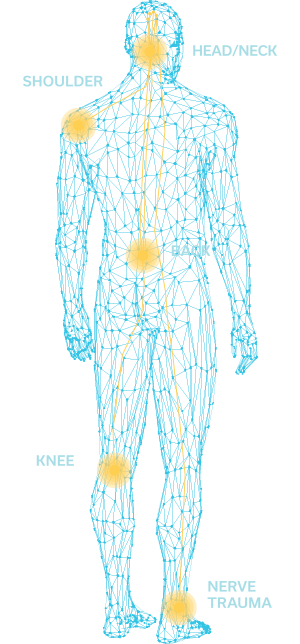
The SPRINT PNS System has been used extensively for low back pain, shoulder pain, knee pain, head and neck pain, post-amputation pain, and chronic and acute post-operative pain, and is cleared for use up to 60 days.

Over 70% of patients reported significant & sustained pain relief
Designed to Outsmart Pain™
The SPRINT PNS System is uniquely designed to selectively and robustly stimulate targeted peripheral nerve fibers for up to 60 days, modulating central plasticity to enable significant and sustained pain relief.
SPR Therapeutics, Inc has been awarded more than $30 million in grants and contracts by the National Institutes of Health and the Department of Defense, allowing us to advance the largest body of PNS-specific research to date.
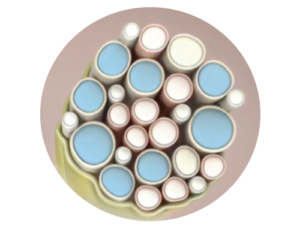
Highly targeted:
Focuses stimulation on targeted nerve fibers to maximize therapeutic benefit and modulate central plasticity.
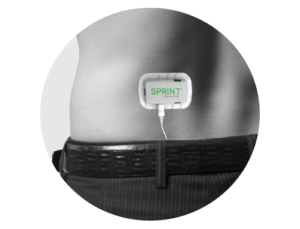
Remote stimulation:
Our patented approach is designed to enable comfortable yet robust activation of nerve fibers to promote central plasticity while avoiding activation of non-target pain fibers.

“A 60-day percutaneous treatment that delivers long-term relief without a permanent implant…this is a game changer.”
Peter Staats MD, MBA, DABA, ABIPP, FIPP
Chief Medical Advisor, SPR Therapeutics
Rethink Your Pain Strategy™
The SPRINT PNS System is a patient-preferred, low risk, alternative to more invasive treatments such as ablation, permanent implants or surgery. The SPRINT PNS System is proven to provide significant and sustained pain relief when prior treatments such as opioids or injections have failed.
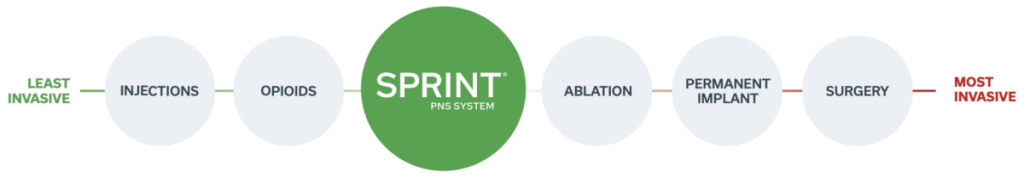
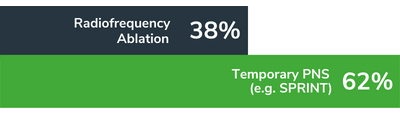
Patient Preferred.
Our system was recently ranked as the most desirable treatment for chronic pain when compared to radiofrequency ablation and permanently implanted neuromodulation systems.
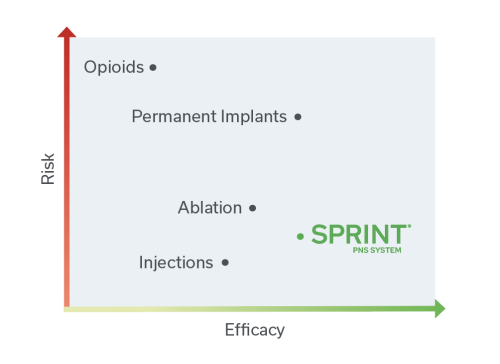
Low risk. High reward.
Compared to other treatment options, the SPRINT PNS System provides highly effective pain relief without a permanent implant, nerve damage or the risk of addiction.
Learn how to help even more patients
Ready to champion a new protocol of care?

